PRODUCT IDENTIFICATION
H.S. CODE
CLASSIFICATION
Disinfectant, Antiseptics, Urea, Peroxide, Oxidizing agent
EXTRA NOTES
Urea peroxide 11% in an anhydrous gel base for treatment of gingival hyperplasia(dilantin induced); cerumenolytic combination of this cpd. in anhydrous glycerol; tooth whitening agent; anti-infective, topical (dental).
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
http://portal.acs.org/
Carbamide peroxide is a combination of urea and hydrogen peroxide that is widely
used at low concentrations (~10%) to whiten teeth. Upon contact with water,
oxygen is released, providing the bleaching (oxidizing) power.
http://www.sigmaaldrich.com/
Application:Peroxide
used with N-halosuccinimides in an efficient α-halogentation of
carbonyl compounds. Used with methyltrioxorhenium (412910) in a
mild oxidation of imines to nitrones Also used to oxidize iodoarenes
to hypervalent iodine compounds
http://www.greenfacts.org/
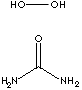
Carbamide peroxide,
(CH4N2O·H2O2), is a chemical that
contains hydrogen peroxide and urea – an organic compound. Pure carbamide peroxide has the form of white crystals or crystal powder, is
soluble in water, and contains approximately 35% hydrogen peroxide. Carbamide peroxide is used as bleach or disinfectant in consumer products
such as hair bleaches, hair perming products, hair relaxers, ear drops,
antiseptic mouth washes, products to treat mouth sores, toothpastes, and tooth
bleaching. It is also used in solutions for the disinfection of contact lenses
and wounds.
Local:
Urea
hydrogen peroxide is
an unstable combination of urea and hydrogen
peroxide in equal amounts. It is soluble in water,
alcohol, and ethylene glycol. It decomposes
at 75-85 C or by moisture. is
used as a source of water-free hydrogen peroxide.
It is used in the bleachings and deodorizing for
fibers. It is used as a disinfectant in cosmetics,
detergents and pharmaceuticals. Its applications
include starch modification, color developer and
catalyst of organic synthesis.
Hazards Overview: Danger! Strong oxidizer. Contact with other material may cause a fire. Corrosive. Light sensitive. Moisture sensitive. Causes burns by all exposure routes. Heat sensitive. Target Organs: Respiratory system, eyes, skin.
APPEARANCE
35.0% min
600 - 700 g/l
TRANSPORTATION